Print version of this lesson plan (https://www.guampedia.com/wp-content/uploads/2020/11/Cone-Snail-Chemistry.pdf).
Subjects
Earth Science, Chemistry, Ecology
Grade-level
Elementary, 2-5
(Special Note: K-5th Grade Science Curriculum.*)
*For kindergarten classes, it is recommended to perform only Day 3 of this lesson plan module.
Time required
60 minutes for 3 or more days
(Additional time is required for homework assignments and assessment tools)
Materials required
- Pencil and paper
- Lab coats
- Goggles
- Nitrile gloves
- Plastic vials (50mL and 15mL)
- Plastic droppers (labeled water and vinegar, to avoid contamination)
- Small funnels (optional)
- Mortar and pestle
- Eye droppers
- Water
- White Vinegar
- Oil
- Kool-Aid packets
- Sand
- Chalk
- Sugar
- Cone snail shells
- Glass Sponge
- Crab shells (preferably from blue or dungeness crabs)
- Various mollusk shells for taxonomy classification
- White board
- Tape
- Rock (s) (optional)
* Biological materials (cone snail shells, crab shell, sponge, shell kits, etc), eye droppers and tubes can be obtained by contacting Dr. Laura Biggs, [email protected].
Related background reading
Let’s Do Science textbook* (http://www.guampedia.com/wp-content/uploads/2010/01/Let27sDoScience_Reduced2.pdf)
*Note: Copies for related reading can be requested from Dr. Laura Biggs, [email protected].
Related documents
A Pre and Post Molecule Building Test: http://www.guampedia.com/wp-content/uploads/2010/01/pre26posttests.pdf
Molecule Building Worksheets: http://www.guampedia.com/wp-content/uploads/2010/01/MoleculeBuilding21.pdf
Molecule Building Worksheets Answer Key: http://www.guampedia.com/wp-content/uploads/2010/01/MoleculeBuilding21answerkey.pdf
Related links
The Cone Snail: http://theconesnail.com
Content Standards
This lesson fulfills various aspects of the following Guam Department of Education Standards depending on grade level: 1st through 3rd and 7th grades.
Lesson Plan
Description
The following lesson plan aims to address the following aspects of the Guam Public School System elementary science core curriculum.
Emphasis
Chemistry investigations of naturally occurring materials found in our kitchens and on our beaches.
Objectives
- Students will be able to identify that vinegar and water differ in their chemical properties.
- Students will be able to understand what a chemical reaction is and be able to give a written or oral example of one.
- Students will be able to understand that objects containing calcium carbonate will react to vinegar.
- Students will be able to classify organisms based on their appearance (taxonomy).
- Students will be able to demonstrate an understanding, through example, of the process of the scientific method.
Day 1: Things Dissolve! And Sometimes They Don’t!
Goals
- Identify that some materials will dissolve in water, while others will not.
- Understand that the composition of the materials may dictate their ability to dissolve.
- Identify that calcium carbonate is a molecule that is present in chalk
- Recognize that the bubbles observed in the experiment are the result of a chemical reaction that breaks down calcium carbonate to form water and carbon dioxide.
- Understand that the world and everything in it is made of molecules.
Teacher Notes
The first day will focus on the idea that:
- Things dissolve.
- We, as scientists, can cause and observe (with our naked eye) chemical reactions.
* It is best, if possible, for students to work in small lab group of 4-5 students (max). Parent or school volunteers could be employed to orchestrate activities within each group.
A bit more about dissolving
A basic principal involved in dissolving is something scientists refer to as Brownian Motion. These are just fancy words to describe the idea that atoms are constantly and randomly moving. This concept can be observed with a simple experiment. Take a clear container full of water and add several drops of food coloring. Without stirring or disturbing the container, observe what happens. Naturally, the food coloring will incorporate into the water. This is because all of the molecules of water are constantly moving, or stirring, and once the food coloring is added it moves along with the water molecules.
The concept of Brownian Motion also comes into play when we add sugar (or other substrate) to water (or vinegar for that matter). Given enough time, the sugar will dissolve into the water to the point of saturation, meaning no more sugar molecules can dissolve in the given amount of water.
Sugar is a relatively hydrophilic molecule, which means that it likes water, often more than it “likes” itself. When sugar is added to water the bonds between molecules of sugar are broken and reformed with water. This does not change the sugar, but instead each sugar molecule temporarily bonds to water. We know that the sugar has not changed because if we were to remove the water (by boiling the solution for example), we would be left with sugar crystals. This is also an easy experiment you can do in the classroom with a portable gas grill, pot of water, and sugar. Alternatively, given enough time, you could also let the container of water sit and over time the water will evaporate leaving the sugar behind.
Chemical reactions occur all around us. We probably create chemical reactions more than we think. For example, when making shrimp kelaguen, vinegar is used to cook the meat of the shrimp. The acidic vinegar, breaks the protein bonds in the meat and reforms the bonds in the same way that heat does. This is a chemical reaction that results in the physical change of the molecules involved. The chemical reaction that is caused in this lesson plan and accompanying text also use vinegar as a catalyst. Below is the chemical reaction that is cause when calcium carbonate and vinegar are added together:
CaCO3 (calcium carbonate) + 2(CH3COOH (vinegar)) = CO2 (carbon dioxide) + H20 (water)
This equation states that if you take one molecule of calcium carbonate and at least 2 molecules of vinegar, you will get carbon dioxide and water. The substances that we are using that have calcium carbonate are chalk, sand (not all types of sand though!), cone snail shell, and crab shell. Therefore when combined with vinegar a chemical reaction is formed and the result is the release of water and carbon dioxide. Carbon dioxide forms gas in the solution manifesting as bubbles rising vigorously from the bottom of the tube. These carbon dioxide bubbles are similar to those that we see in soda, however the carbonated bubbles in soda are not a chemical reaction, they are merely carbon dioxide gas trapped in the can escaping when the pressure is released.
Procedure
Lesson Plan Mechanics
- Prior to “Let’s Do Science” experiments beginning, students should have read up to page 14 (Experiment One) and also be asked to list at least five questions they have about anything relating to the book, science, etc. These questions should be collected on Day 1 and can be used to customize Day 3 of the module.
- Front of Class Introduction: Teacher has three large plastic containers each about half full with water. In addition the teacher has a packet of Kool-Aid, Oil, and a rock(s). Pose questions to students and place their answers into chart form on the board:
- Can we identify differences between these three objects?
- Do we know what will happen if I put this Kool-Aid into water?
- Respond to answers from students and dump Kool-Aid in water.
- Do we know what will happen if I put some oil into water?
- Respond to answers and dump oil into water.
- Do we know what will happen if I put a rock(s) into water?
- Respond to answers and dump rock(s) into water.
- What if I have something that is solid and I break it up?
- Instruct students to form into their groups (around one table or set of desks). Instruction from this point on will be on a group level to eliminate screaming above the whole class. Each group will be assigned an adult facilitator and a group name. The students will take turns dispensing either water or vinegar into the appropriate tubes. Each student will have their own lab worksheets (Note: photocopies from pages 14-21 of “Let’s Do Science”).
- Teacher and students will make predictions about what they think might happen when water and vinegar is added to each of the vials. Students will come up with hypotheses for each of their experiments, which should be recorded on their worksheet. After predictions have been made, using the eye droppers, teachers will instruct students to place water and vinegar in each of the containers with sand, chalk and sugar. Students will record observations in their worksheet.
Conclusion
- Discuss why the different materials responded the way they did when placed in the water.
- Why? Because of their composition… what they’re made of!
- What happens when we use a solution other than water? Vinegar?
- Summarize results from all of the groups on the board.
- Prepare materials for the following day (if time allows, if not this can be done at the beginning of Day 2).
- Using the mortar and pestle, grind up crab shell, glass sponge. Store in 15mL tube for tomorrow.
Day 2: Biological Materials Have Different Biochemical Profiles
Goals
- Understand that biological materials behave differently in water and vinegar.
- Understand that it is the composition of the biological materials that makes them behave differently.
- Identify that calcium carbonate is a molecule that is present in cone snail shell, crab shell and chalk.
- Recognize that the bubbles observed in the experiment are the result of a chemical reaction that breaks down calcium carbonate to form water and carbon dioxide.
Procedure
- Instruct students to form into their groups. Instruction from this point on will be on a group level to eliminate screaming above the whole class. Each group will be assigned an adult facilitator and a group name. The students will take turns dispensing either water or vinegar into the appropriate tubes. Each student will have their own lab worksheets (Note: photocopies from pages 14-21 of “Let’s Do Science”).
- Teacher and students will make predictions about what they think might happen when water and vinegar is added to each of the vials. Students will come up with hypotheses for each of their experiments, which should be recorded on their worksheet. After predictions have been made, using the eye droppers, teachers will instruct students to place water and vinegar in each of the containers with crab shell, glass sponge, cone snail shell. Students will record observations in their worksheet.
Conclusion
- Tic-Tac-Toe Biochemistry. Introduce the common element that is present in all things that bubbled in their experiments – Calcium Carbonate! This is also found in our bones…. And it reacts with an acid (vinegar)
- Class Activity – On chalkboard summarize what students observed (vinegar only) by writing things that dissolved and bubbled in RED while writing things that did not dissolve in BLUE.
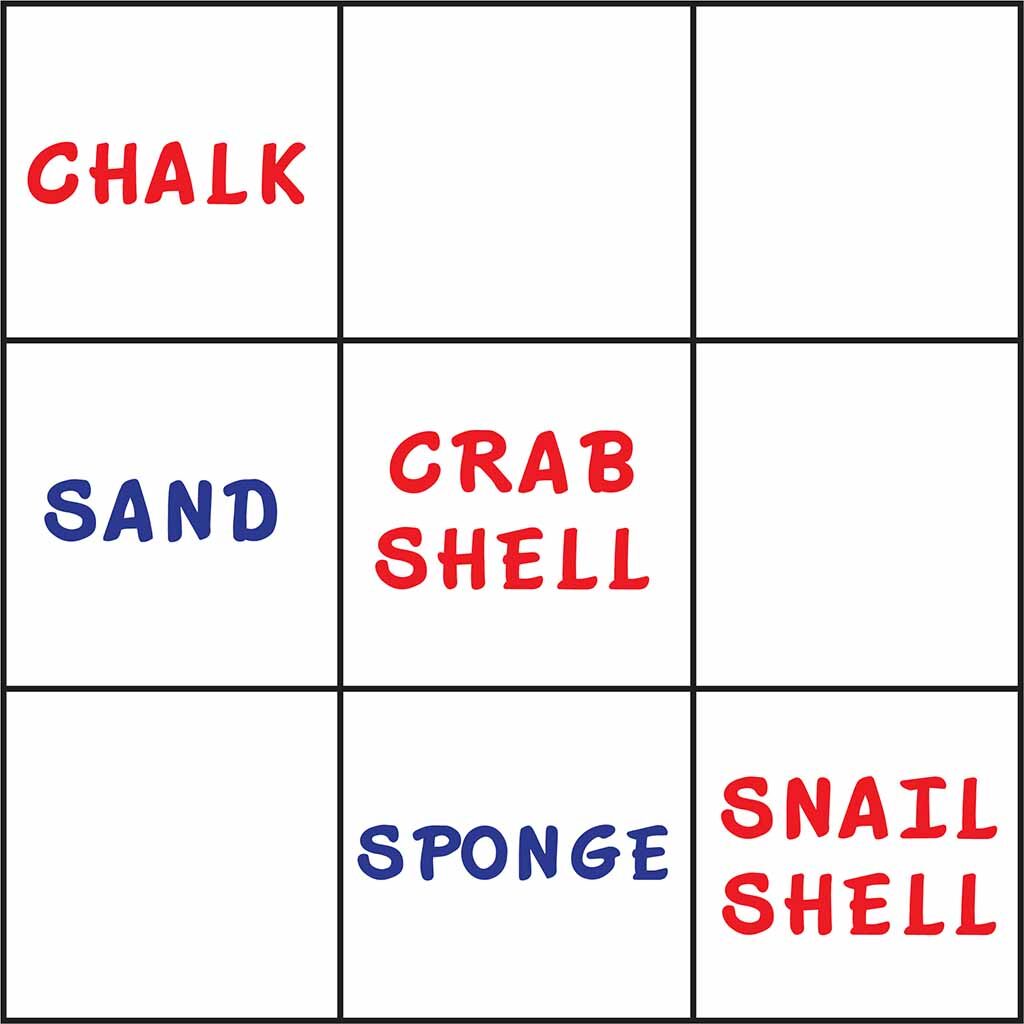
Teacher Notes
Taxonomy is the study of how things are related. Taxonomy help scientists make a family tree for all the plants and organisms. Scientists use many things to determine how things are related, the major source of information currently being DNA, the genetic material of all organisms. By looking at the genetic makeup of DNA, scientists can determine where plants and organisms lay on their family tree.
Day 3: Taxonomy and Biology
Procedure
Warm-Up
Start with a quiet short answer response.
- Hand out paper for kids to fill-in their responses to the following questions:
- I learned something really interesting about science and that was:
- What causes the different substances to bubble?
- Why didn’t the glass sponge bubble?
- Something I’m dying to know is:
- (or other adapted questionnaire)
Introduction
- Facilitate kids into coming up with categories to sort their shells.
- How do we tell things are different? Size? Shape? Colors? Etc.
- Distribute snail shell kits
- Have students organize the shells by categories. Students will work together in their groups and tape the shells to a piece of cardboard. Share with the class.
- Cone Snail Demonstration
- Video of snails eating.
- Show snails in tanks.
Conclusion Activity
- Answer questions the students have about marine species (or just cone snails).
- Take-home assignment.
Tests and Worksheets
A Pre and Post Molecule Building Test: http://www.guampedia.com/wp-content/uploads/2010/01/pre26posttests.pdf
Molecule Building Worksheets: http://www.guampedia.com/wp-content/uploads/2010/01/MoleculeBuilding21.pdf
Molecule Building Worksheets Answer Key: http://www.guampedia.com/wp-content/uploads/2010/01/MoleculeBuilding21answerkey.pdf
Editor’s note: Funding for this lesson plan was provided by the Howard Hughes Medical Institute, Olivera Lab, University of Utah. Please email Dr. Laura Biggs at [email protected] with questions or comments.
